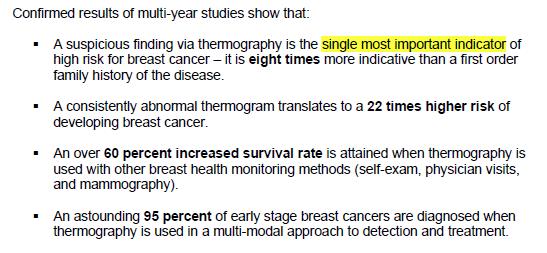
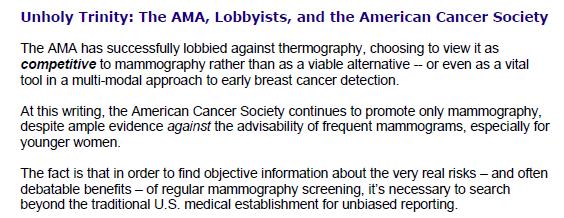
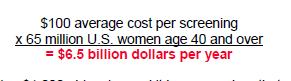The use of animals for medical research is a sensitive and complex topic. While the majority of people in the UK understand and support the need for animal experiments to advance medical research, many may not be aware of the united front within the research community to ensure that these experiments are carried out to the highest standards. The National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) is leading this movement to help researchers and research funders improve animal experimentation, and ultimately produce better science.
The 3R’s stand for Replacement, Refinement and Reduction, and identify the key ways research can be improved when it involves the use of animals. Replacement encourages research which seeks to develop or utilise non-animal methods, refinement identifies ways in which experimental methods can be produced to improve animal welfare, and reduction encourages better study design to reduce the number of animals used in research. The 3R’s are adopted by funding bodies such as medical research charities, the Medical Research Council and the Wellcome Trust and adoption of the principles is required before funding is released.
Making science better
Adhering to and promoting the 3R’s is not just about protecting the animals, it’s about making science better, more efficient, and more reproducible, so that the animals used in experiments are not wasted. We should be asking researchers to disclose full details of their animal work not only so we can scrutinise how well they’ve treated their animals but also so we can see that the work is justified.
There are several things a researcher can do to adhere to the 3R’s. Carefully planned and well thought out experimental design can reduce the sample size of animals needed. Asking for advice from a statistician when designing protocols can identify areas where numbers of animals can be reduced through accurate prediction of animal numbers needed and power calculations. Understanding what other statistical options are available could also reveal more effective ways to conduct experiments. There also needs to be in place plans to minimise experimental bias to enhance the quality of research and prevent over-use of animals to achieve robust results. This could be as simple as blinding the analysis of animal tissue to investigators, ultimately producing better data sets with fewer animals.
Reporting for reproducibility
In 2010, the NC3Rs published a paper in PLoS Biology highlighting the need for better reporting of animal research in published studies. The statistics they report are alarming, only 59% of 271 randomly selected articles stated a research objective and the number of animals or species used, 87% did not report randomising their studies and 86% did not report any blinding to reduce bias. Only 70% properly reported statistics and presented the results with a measure of precision or variability.
The solution was to develop the ARRIVE guidelines for the reporting of in vivo experiments. The ARRIVE guidelines are essentially a checklist for researchers to follow when reporting animal research. Animal experiments can be written up in great detail and submitted to journals as supplementary material should there be content limits set by the editors. It’s imperative that research funders demand this full disclosure and transparency from their grant holders so that maximal output is achieved from animal experiments, work is reproducible and the need for excessive animal use is avoided.
Transparency
As discussed above, transparent reporting of animal research is crucial for accuracy and reproducibility of experiments. This transparency ensures that any deviation from what the researcher expects of their experiment can be picked up and resolved before any unnecessary experiments are carried out. But there also needs to be transparency in how the animals were developed for experimentation. For example, if a researcher is developing their own mouse models or is maintaining mouse models for an extended period of time, they need to be reporting phenotype and genotype data to pick up any deviation in the animals background. Genetic drift occurs rapidly in mouse breeding programmes with over 100 SNP’s appearing in the mouse genome in one generation. Without full reporting the mouse phenotype may not be reproducible due to the emergence of sub-strains over time.
The 3R’s for a better future
Animal testing is currently necessary for new treatments to be developed for diseases such as cancer and dementia. Legal regulations require that new treatments are shown to be safe and effective in at least two different animal species before they can be approved for clinical trials in humans. It is not practical to ban animal testing for medical research as we don’t currently have the methods or alternatives to remove these strict but necessary regulations on drug testing. The work by the NC3Rs is ensuring that where these experiments have to take place, they are being done to maximise the use of each animal, to produce data which has the biggest impact and maintain the highest level of animal welfare. On top of this they are promoting and funding work into the replacement of animal models with non-animal alternatives so that we can move towards a future without the need for animal experimentation. It’s important to remember that it’s not solely about a reduction in numbers of animals being used but a refinement of the techniques, husbandry and reporting, which will ultimately lead to better science.